A nuanced understanding of the registration process and the regulatory landscape is imperative. The Health Sciences Authority (HSA) plays a pivotal role in overseeing medical devices, ensuring they meet stringent safety, efficacy, and quality standards. This step-by-step guide aims to demystify the intricacies of medical device registration in Singapore.
Step 1: Understand the Regulatory Landscape
Before delving into the medical device registration process, it is crucial to familiarise oneself with the regulatory framework in Singapore. The HSA stands as the primary regulatory authority, committed to ensuring that medical devices adhere to the highest standards before reaching the public.
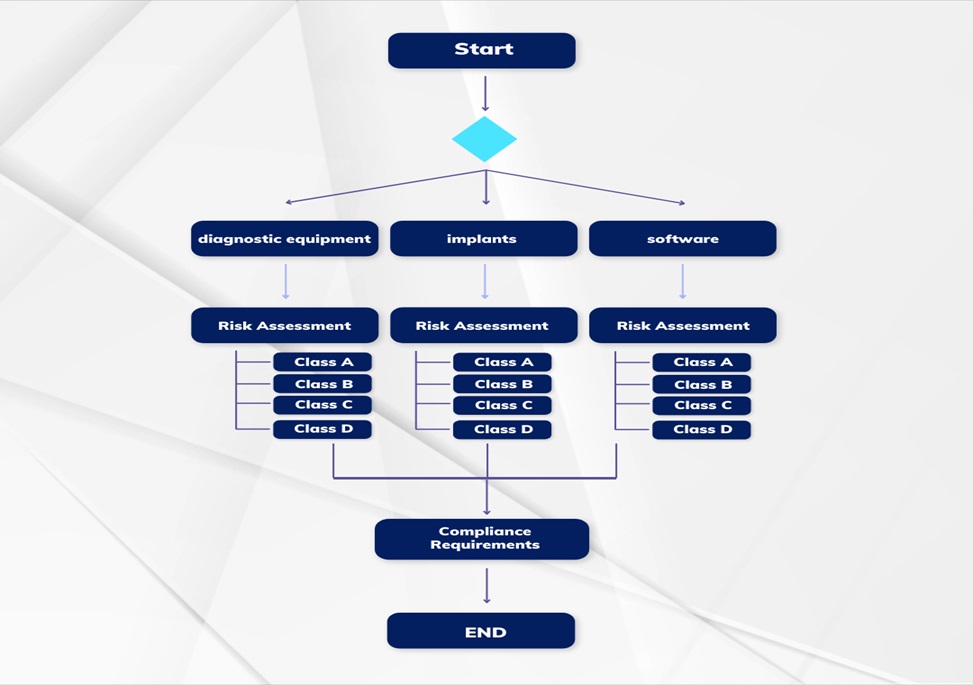
Step 2: Determine the Device Classification
The classification of medical devices sets the tone for the regulatory journey, influencing the requirements and processes to be followed. The HSA classifies devices into four main classes – Class A, Class B, Class C, and Class D, each indicating a different level of risk. Accurately classifying the device streamlines subsequent registration processes.
Step 3: Appoint a Local Authorised Representative
For foreign manufacturers, a local authorised representative is a mandatory liaison between the manufacturer and the HSA. This representative facilitates communication, ensuring compliance with local regulations and playing a crucial role in the overall success of the registration process.
Step 4: Compile Essential Documentation
Thorough documentation is the cornerstone of a successful medical device registration. Manufacturers must prepare a comprehensive Technical File or Design Dossier containing information about the device’s design, manufacturing processes, risk assessments, and conformity to standards. Supporting documents such as proof of the quality management system and clinical data are also essential.
A
ALSO READ: Understanding the Medical Device Registration Process in Singapore
Step 5: Submit a Medical Device Registration Application
Armed with a complete set of documentation, manufacturers can submit a Medical Device Registration application to the HSA. This application should include the device’s classification, intended use, and a detailed summary of safety and performance data. Timely submission is critical for a smooth regulatory process.
Step 6: HSA Review and Approval
The HSA conducts a thorough review of the submitted application, assessing the device’s safety, efficacy, and quality. This process may involve multiple rounds of evaluation, with manufacturers expected to address any queries or provide additional information as requested. Upon approval, the HSA issues a Letter of Approval, allowing manufacturers to market and distribute the medical device in Singapore.
Step 7: Post-Market Obligations
Approval does not mark the end of responsibilities. Manufacturers must actively monitor the device’s performance, promptly address safety concerns, and adhere to ongoing reporting requirements. Regular communication with the HSA ensures manufacturers stay informed about updates or changes in regulatory requirements.
Step 8: Labelling and Packaging Compliance
Compliance with local regulations regarding labelling and packaging is essential. Manufacturers must provide clear and accurate information, including usage instructions, precautions, and contact details for the local authorised representative. Meeting these requirements enhances the device’s marketability and ensures adherence to regulatory standards.
Step 9: Post-Market Surveillance
Post-market surveillance involves continuous monitoring of the medical device’s performance and safety after it has been introduced to the market. Manufacturers should establish a robust surveillance system to collect and analyse data on the device’s real-world usage. This step ensures prompt identification and response to emerging safety concerns, contributing to ongoing regulatory compliance.
Step 10: Stay Informed About Regulatory Updates
Given the dynamic nature of the regulatory landscape, manufacturers must stay informed about any updates or modifications. Regularly checking the HSA’s official website and participating in industry forums provide valuable insights into evolving regulatory requirements, enabling manufacturers to adapt their processes accordingly.
Conclusion
Successfully navigating the medical device registration process demands a comprehensive understanding of the regulatory framework and meticulous attention to compliance. Engaging in regulatory affairs in Singapore is not just a legal requirement; it is a commitment to the health and well-being of the consumers who rely on these medical devices for their care.
Contact Reg Consultants to learn more about medical device registration so you can apply for your business.